2. An orbital formed from overlap of two atomic orbitals is like an individual atomic orbital in that it is full when two paired electrons occupy it.
3. This overlap is not possible because it would include three electrons occupying one shared orbital.
5 (a) a digram
(b) The angle for the H—S—H bonds is predicted to be 90°, since that is the angle between the two p orbitals of the
sulfur that are involved in the bonding.
(c) The prediction is close to the measured angle and therefore inconclusive. The experimental uncertainty of the
measured 92º is required to see if the small difference can be explained by the quality of the measurements. If
not, then the valence bond theory may require a revision or adjustment.
8. (a) one 1s orbital
(b) one 2p orbital
(c) two 3p orbitals
(d) one 4p orbital
9. Two electrons are present in an orbital formed from overlap.
10. (a) ground state—1s2 2s2 2p2 promoted state—1s2 2s1 2p3 sp3 hybridization
(b) ground state—1s2 2s2 2p1 promoted state—1s2 2s1 2p2 sp2 hybridization
(c) ground state—1s2 2s2 promoted state—1s2 2s1 2p1 sp hybridization
13. Hybridized orbitals are formed only during bonding—they do not exist in isolated atoms.
14. The hybridized orbital concept was necessary to explain the similarity of bonds and the observed bond angles in structures like CH4, and to explain the formation of double and triple bonds.
17. (a) PCl5 requires five hybrid orbitals, and SF6 requires six, using their valence, from the given formulas for their
compounds. Using promotion of electrons in orbital configurations, we obtain the same answer.
(b) Using promotion of electrons in orbital configurations:
P atom: 1s2 2s2 2p6 3s2 3p3 to 1s2 2s2 2p6 3s1 3p3 4d1
S atom: 1s2 2s2 2p6 3s2 3p4 to 1s2 2s2 2p6 3s1 3p3 4d2
(c) Scientists know the directions of hybrid orbitals from the molecular shape, which can be determined by spectroscopy
and X-ray diffraction.
29. Hypothesis
(a) One might logically hypothesize that double bonds should be longer and stronger than single bonds, and triple
bonds longer and stronger yet, because more electron orbital density is between the nuclei. More electron density
might make the bonds longer with more space required for more electrons but make the bond stronger because
there is more electrostatic attraction of electrons and nuclei.
Analysis
(b) According to the evidence provided, the order of bond length, from shortest to longest is triple, double, and then
single bonds. The order of bond strength, from weakest to strongest is single, double, and then triple bonds. Single
bonds are the longest but the weakest, and triple bonds are the shortest but the strongest.
Evaluation
(c) The hypothesis is verified for bond strength, but not for bond length. The reasoning based on electron density
appears acceptable for bond strength but does not appear correct for bond lengths. (Note that the reasoning did
not take into account the shapes and directions of the p orbitals forming the pi bonds.)
2. Is a diagram
3. (a) PO43– will be tetrahedral in shape because it has four bond pairs around the P atom.
(b) IO3– will be pyramidal in shape, having three bond pairs and one lone pair around the I atom.
4. (a) According to VSEPR theory, the shape around each carbon atom in cubane should be tetrahedral, since there are
four bond pairs around each.
(b) If we assume an ideal cubic shape, three of the bond angles around each carbon have to be 90o.
(c) The normal tetrahedral angle is about 109o. To make these bonding orbitals bend to about 90o would greatly
increase the repulsion of the electron pairs. This stress likely makes this molecule very unstable.
10. To make the rules of VSEPR theory work, multiple bonds must be treated just like single bonds—that is, they are considered to be one bond, which involves 4 or 6 electrons.
11. (a) linear
(b)linear
(c)trigonal planar 1st two carbons, tetrahedral on the third carbon
(d)linear 1st two carbons, tetrahedral on the third carbon
(e)trigonal planar
(f)linearV
12. VSEPR is a very successful scientific theory. It successfully predicts the shapes of most molecular structures with a minimum of complexity—both being criteria for a “good” theory.
3. The list of the bonds in order of increasing bond polarity is assumed to be the same as the order of increasing difference
in electronegativity of the bonded atoms. Thus, the orders are as follows:
(a) H—H, C—H, Be—H, N—H, Li—H, O—H, F—H
(b) I—I, I—Cl, P—Cl, (Li—I, Al—Cl), Rb—F (Note: Li—I and Al—Cl have equal dipoles.)
(c) C—H, C—O, O—H
(d) C—H, C—Cl, C—F
For Questions 6, 7, & 8 Click Here
(Page 256) Understanding Concepts
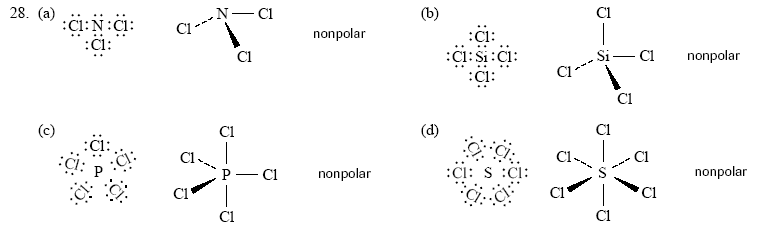
1. (a) Beryllium bromide is nonpolar because it is symmetrical. Therefore, the bond dipoles add up to zero for the whole
molecule.
(b) Nitrogen trifluoride is polar because it has polar bonds and is not symmetrical. The sum of all of the bond dipoles
produces a non-zero dipole for the whole molecule.
(c) Methanol is polar because it has polar bonds and is not symmetrical. The sum of all of the bond dipoles produces
a non-zero dipole for the whole molecule.
(d) Hydrogen peroxide is nonpolar because it has polar bonds and is assumed to be symmetrical. The sum of all of
the bond dipoles produces a zero dipole for the whole molecule. (Note that this answer ignores any rotation about
the O-O bond and considers only the most stable arrangement.)
(e) Ethylene glycol is nonpolar because it has polar bonds and is symmetrical. The bond dipoles have a zero resultant.
(Again, this assumes the most stable arrangement.)
2. (a) hydrogen fluoride; the H-F bond is more polar (electronegativity difference is greater)
(b) chloromethane; the C-Cl bond is more polar (electronegativity difference is greater)
(c) nitrogen tribromide; the N-Br bonds are more polar (electronegativity difference is greater)
(d) water; the O-H bonds are more polar (electronegativity difference is greater)
3. (a) ethane; because it has 8 more electrons (and protons) than methane
(b) oxygen; because it has 2 more electrons (and protons) than nitrogen
(c) sulfur dioxide; because it has 18 more electrons (and protons) than nitrogen dioxide
(d) Methane and ammonia are isoelectronic, with 10 electrons each. They should have equal-strength London forces.
4. (a) oxygen difluoride; beryllium difluoride is nonpolar (no dipole–dipole forces) and also has fewer electrons
(weaker London forces).
(b) chloromethane; ethane is nonpolar (no dipole–dipole forces) and also has fewer electrons (weaker London forces).
10. a) For diagram see note on Intermolecular Forces
(b) The actual boiling points for water and ammonia (compared to an estimate from the graph) are about 170oC and
70oC higher, respectively.
(c) The actual boiling points are much higher for both water and ammonia because of hydrogen bonding between
molecules in these substances.
(d) Likely, the difference is much greater for water (than for ammonia) because oxygen is a more electronegative
central atom than nitrogen; and possibly also because of differences in molecular shape. Water molecules have
more free lone pairs available for hydrogen bonding; and in ammonia, the third bond dipole acts to partially
cancel the other two bond dipoles.
11. Water beading on a surface means that the surface material must have very low intermolecular attraction for water
molecules. This would mean no polar areas on the surface molecules, and certainly no hydrogen bonding locations.
Page 266 Questions
1. (a) London forces
(b) hydrogen bonding, dipole–dipole and London forces
(c) dipole–dipole and London forces
(d) hydrogen bonding, dipole–dipole and London forces
(e) dipole–dipole and London forces
(f) hydrogen bonding, dipole–dipole and London forces
(g) hydrogen bonding, dipole–dipole and London forces
(h) dipole–dipole and London forces
(i) dipole–dipole and London forces
2. The very high solubility of ammonia in water is due to the high number of hydrogen bonding sites (see diagram).
Every ammonia molecule can hydrogen bond at least four times, as can every water molecule in the solution.
3. (a) 2-chloropropane should have low or medium solubility, because it is polar and water is also polar.
(b) 1-propanol should have high solubility, because it is not only polar but can hydrogen bond with water molecules.
(c) Propanone should have medium solubility, because it is quite polar, and so is water.
(d) Propane should have low solubility, because it is a nonpolar substance and water is polar.
4. (a) Bromine should have stronger intermolecular attractions. Both molecules are nonpolar but bromine has larger
molecules with a greater number of electrons, so it should have the stronger London force.
(b) Hydrogen chloride should have stronger intermolecular attractions. Hydrogen chloride and fluorine are isoelectronic
which means the London force should be the same. However, HCl has polar molecules so it should have
additional dipole–dipole force.
(c) Ammonia should have stronger intermolecular attractions. Ammonia and methane are isoelectronic so the
London force should be the same. Unlike methane, ammonia is polar and has hydrogen bonding. Ammonia therefore
has additional attractions, dipole–dipole force, and hydrogen bonds.
(d) Water should have stronger intermolecular attractions. Both molecules are polar but hydrogen sulfide is less polar.
Although hydrogen sulfide has a greater number of electrons and stronger London forces, water has hydrogen
bonding. This is likely much more significant than the difference in London forces.
(e) Silicon tetrahydride should have stronger intermolecular attractions. Both substances are nonpolar and silicon
tetrahydride has more electrons per molecule, so it should have more London force.
(f) Ethanol should have stronger intermolecular attractions. The two substances are isoelectronic which means the
London force should be the same. Both are polar but ethanol has hydrogen bonding and chloromethane does not.
5. Ethanol should have the greater surface tension because it has the stronger intermolecular attractions. Propane and ethanol molecules are isoelectronic so the London force is the same for both. There are no other intermolecular attractions between propane molecules because they are nonpolar. However, ethanol has additional dipole–dipole and hydrogen bonds between its molecules.
2. (a) covalent bonding covalent network crystal
(b) ionic bonding -- ionic crystal
(c) covalent bonding -- molecular crystal
(d) covalent bonding -- covalent network crystal
(e) metallic bonding -- metallic crystal
(f) ionic bonding -- ionic crystal
3. The melting point of a solid is proportional to the attractive forces between the entities of the solid. Strong bonds like covalent bonds will result in very high melting points, while much weaker bonds like London forces will result in lower melting points.
4. Metals are generally malleable, ductile, and flexible because the bonding between atoms in metals is nondirectional— so changing the position of the atoms (shape of the solid) does not “break” the bonding.
5. (a) Aluminum is a light, soft, flexible, silvery metal solid, with a fairly low melting point for metals. Aluminum
oxide is a very hard network crystalline solid with an extremely high melting point. In aluminum, the bonding is
metallic bonds of a lower-than-average strength for metals. In aluminum oxide, very strong ionic bonds lock the
aluminum and oxygen ions in a rigid three-dimensional network.
(b) Carbon dioxide is a soft molecular solid with a very low boiling point. Silicon carbide is a very hard network
crystal solid with an extremely high boiling point. In carbon dioxide, the molecules are held together by relatively
weak nondirectional London forces only. In silicon carbide, the silicon and carbon atoms are locked in a threedimensional
network by very strong covalent bonds.
7. (a) high melting point, conducts electricity vanadium, V(s)
(b) low melting point, soft phosphorus pentoxide, P2O5(s)
(c) high melting point, soluble in water sodium bromide, NaBr(s)
(d) very high melting point, non-conductor silicon dioxide, SiO2(s)
Page 276 Questions
1. Ionic substances do not conduct while solid because all ions are locked in position. Upon melting, the ions are free to move and the substance conducts freely. In aqueous solution, the ions are also free to move and the solution will conduct more or less well, depending on concentration.
2. A substance conducts electricity when its particles have a charge and are free to move, meaning the particles must be held only by weak forces.
3. In calcium oxide, the ions have double the charge that the ions in sodium chloride have, creating a significantly greater interionic attraction.
4. In solid carbon dioxide, the bonding consists of London forces between molecules. These relatively weak forces result in very low melting and boiling points, and make the solid a soft substance. By contrast, in silicon dioxide, the bonding consists of a continuous network of covalent bonds between atoms. These very strong forces result in very high melting and boiling points, and make the solid a very hard, brittle substance.
5. Most metals have a relatively high density because their atoms are closely packed together in solid form, held together strongly by mobile valence electrons that are dispersed throughout a structure of positive ions.
6. Covalent network structures have the highest hardnesses and also the highest melting and boiling points, indicating that covalent bonds are the strongest. Molecular structures have the lowest values for these properties, indicating that intermolecular bonding forces are the weakest. Metals and ionic compound properties fall between these first two, but the values for both metals and ionic compounds vary widely, depending on the specific substance.
7. Rubbing your zipper with your pencil will coat it with graphite which will act as a lubricant. Graphite crystals form in layers one atom thick held to each other only by very weak London force, so they slide very easily over each other.
8. Diamond is composed of carbon atoms bonded four times each in a three-dimensional covalent network. It is a colourless solid, extremely hard, and a nonconductor. Its main use is as an abrasive; pure crystals are used as gemstones.
Graphite is composed of carbon atoms bonded three times each in a two-dimensional covalent network resulting in layers one atom thick which are held to each other by London force. It is a grey-black solid, very soft and slippery, and a good electrical conductor because of the mobility of the delocalized, unbonded fourth electron of each carbon atom. It has a myriad of uses in industry, most of which have to do with its high melting point and lubricant properties.
9. XCla must be an ionic compound. The high melting point suggests that it has very strong bonding holding the entities together, but is water soluble, so it is not likely a covalent network crystal. YClb must be a molecular compound. The melting and boiling points are low, indicating London forces holding the entities together. The solubility argues that the molecules are nonpolar.

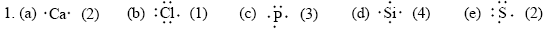
3. (a) three lone pairs
(b) one lone pair
(c) two lone pairs
(d) no lone pairs
(e) one lone pair
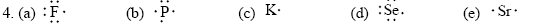
8. (a) sp3
(b) sp2
(c) sp
(d) sp3
9. A sigma bond involves overlap of orbitals directly, or end to end, between the atomic nuclei. A pi bond involves side-by-side overlap of the two lobes of p orbitals above and below a line between the atomic nuclei.
14. (a) linear
(b) trigonal planar
(c) tetrahedral
(d) tetrahedral
(e) linear
(f) V-shaped
(g) tetrahedral
(h) V-shaped (around each O)
15. (a) a diagram (b) In methane, the bond angles are the normal tetrahedral angle. In ammonia, repulsion from the lone pair compresses the bond angles a bit, and in water, stronger repulsion from two lone pairs compresses the bond angle even more.
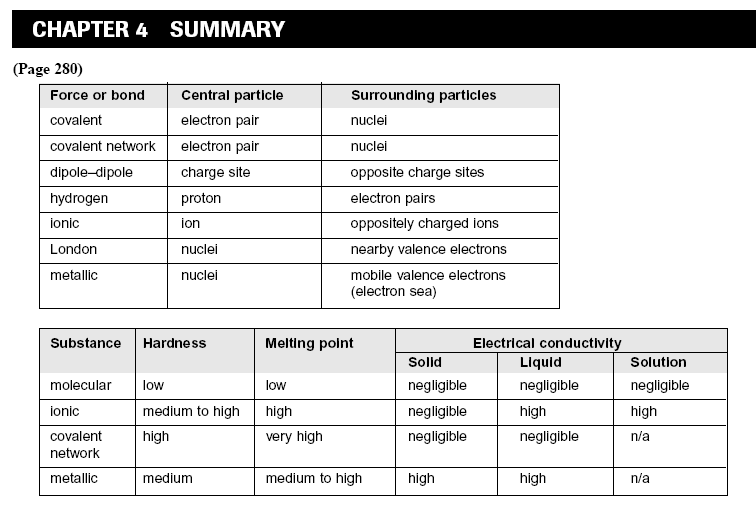
18. (a) BeH2 is a nonpolar molecule because it is linear and symmetrical, so its bond dipoles balance each other. H2S is
a polar molecule because it is V-shaped and not symmetrical, so its bond dipoles combine to produce a nonzero
resultant dipole.
(b) BH3 is trigonal planar, while NH3 is pyramidal in shape, because NH3 has a lone pair of electrons repelling the
three bond pairs.
(c) LiH has a melting point of 688°C because solid LiH has an ionic crystal structure, with ions held together by relatively
strong ionic bonding. HF has a melting point of –83°C because solid HF has a molecular crystal structure,
with molecules held together by much weaker intermolecular forces.
19. The larger molecules have stronger London force intermolecular bonding because the molecules have a greater number of electrons per molecule. Therefore, the larger the molecule in this series, the higher the boiling point.
25. (a) Metallic solids are composed of atoms with mobile valence electrons—they may be thought of as an arrangement
of close-packed positive ions held together by strong mutual attraction for electrons that permeate the structure.
Network solids are composed of atoms held together by very strong (directional) covalent bonds.
(b) Network solids are composed of atoms held together by very strong (directional) covalent bonds. Molecular
solids are composed of molecules held together by relatively weak intermolecular forces.
(c) Molecular solids are composed of molecules held together by relatively weak intermolecular forces. Ionic solids
are composed of positive and negative ions held together by relatively strong (nondirectional) ionic bonds.
20. CH4(g) (–164oC), has London force; NH3(g) (–33oC), has London force, dipole–dipole force, and hydrogen bonding; and BF3(g) (–100oC), has London force. Ammonia has the strongest intermolecular bonds because of the hydrogen bonding; boron trifluoride has London force from a 32-electron molecule; and methane has weaker London force from a 10-electron molecule.
21. (a) Nickel has a much higher melting point than sodium chloride because
the metallic bonding holding nickel atoms
together is stronger than the ionic bonding holding sodium and chloride ions
together.
(b) Solid nickel will conduct well, because the atoms’ valence electrons are free
to move. Solid sodium chloride will
not conduct because the charges (ions) are not free to move.
(c) Solid nickel will not dissolve, because the atoms attract each other much
more than water molecules can attract
them. Solid sodium chloride will dissolve because the charges (ions) are
strongly attracted by polar water molecules.
22. (a) Hexane has London force.
(b) 1-butanol has London force, dipole–dipole force, and hydrogen
bonding.
(c) Ethylamine has London force, dipole–dipole force, and hydrogen
bonding.
(d) Chloroethane has London force and dipole–dipole force.
(e) Calcium carbonate has ionic bonds.
(f) Diamond has convalent bonds.