

The Formula
Coulomb's law describes the force between two charged particles.
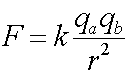
Here, F is the force between the particles, qa and qb are the charges of particles a and b. The separation between the particles is r, and k is a constant, 8.99x109 (Nm2/C2). Note that the force falls off quadratically, similarly to the behavior of the gravitational force. The force is attractive, when F is negative, hence when the charges have opposite sign. Opposites attract - like charges repel. Of course, remember that force is a vector, which in this case points parallel to r.
If a charge a is in the presence of several charges, the force that a feels is the sum of the forces from the remaining charges. For instance if there are three charges, a, b, and c, the net force felt by a is:
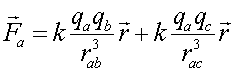
where rab is the separation between a and b.
Analogy between electric and gravitational forces
The electric(Coulomb's Law) and gravitational forces have similar forms:
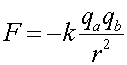
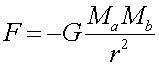
Note that gravitational forces are always attractive (F is always negative), while the electric force is attractive for opposite and repulsive for like charges. Also notice that the electric force is MUCH stronger:
k = 8.99x109 Nm2/C2 G = 6.67x10-11 Nm2/kg2
